LIQUID BASICS
By now you know what a solid is. If you wave your arms around you can find a gas. But what about liquids? Not that we suggest it, but you know you've got some spit. That's a liquid. What about your blood? That's a liquid too. The main thing is to figure out what makes those things liquids.
Liquids are a between phase of matter. They are right between solids and gases. One characteristic of a liquid is that it fills the shape of any container which holds it. So you pour some water in a cup. It fills up the bottom of the cup first and then fills the rest. It also takes the shape of the inside of the cup, starting at the bottom because of GRAVITY. When it is in that cup, it also has a flat surface. That's because of gravity too.
One other characteristic of liquids is that they are very hard to COMPRESS. When you compress something you try and take a certain amount and force it in a smaller space. Solids are tough to compress too, but gases are easy. When you compress something you can squeeze it so the atoms in the substance are closer together. When pressure goes up things are compressed. Liquids already have their atoms close together so it's hard to push them even closer together.
SO YOU WANT TO BE A LIQUID
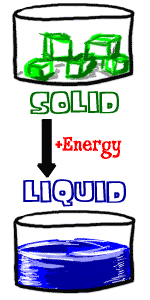 If you want to be a liquid you could start out as two different things. You could be a solid or you could be a gas. Each of them has a different way of becoming a liquid.
If you want to be a liquid you could start out as two different things. You could be a solid or you could be a gas. Each of them has a different way of becoming a liquid.
Let's say you're a solid. That's you, a handsome cube of ice sitting on a counter. All you do is dream of becoming liquid water. What you need is some ENERGY. Atoms in a liquid have more energy than the atoms in a solid. The easiest energy around is probably heat. There is a magic temperature for every substance called the MELTING POINT. When a solid substance reaches the temperature of the melting point, then it can become a liquid. For water the temperature has to be a little over zero degrees celsius. If you were salt or sugar it would be a higher temperature.
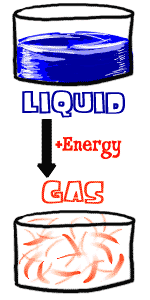 So solids need more energy, the reverse is true if you are a gas. You need to lose some energy from your very excited gas atoms. The easy answer is to lower the surrounding temperature. When the temperature drops, energy will be sucked out of your gas atoms. When you get to the CONDENSATION POINT, that's the temperature when you become a liquid. If you were the steam of a boiling pot of water and you hit the wall, the wall would be so cool that you would quickly become a liquid. So solids need more energy, the reverse is true if you are a gas. You need to lose some energy from your very excited gas atoms. The easy answer is to lower the surrounding temperature. When the temperature drops, energy will be sucked out of your gas atoms. When you get to the CONDENSATION POINT, that's the temperature when you become a liquid. If you were the steam of a boiling pot of water and you hit the wall, the wall would be so cool that you would quickly become a liquid.
EVAPORATION
Sometimes a liquid can just be sitting there and the molecules will become a gas. That's called EVAPORATION. You might be wondering how that can happen when the temperature is low. It turns out that all liquids can evaporate at room temperature and pressure. Evaporation is when there are atoms or molecules escaping from the liquid and turning into a VAPOR. Not all the molecules in a liquid actually have the same energy. The energy you measure is really an AVERAGE of all the molecules. There are always a few molecules with a lot of energy and some with barely any energy at all. It is those with a lot of energy that build up enough power to become a gas and leave the liquid. When it leaves, it has evaporated.
|