SOLID BASICS
Solids can be made up of many things. They can have ELEMENTS or COMPOUNDS inside. They can also be made up of mixtures, or combinations, of different elements and compounds. Most of the solids you see are mixtures. Most rocks are mixtures of many elements and compounds. Concrete is a good example of a man-made solid mixture.
You can learn more about mixtures here...
CHARACTERISTICS OF SOLIDS
First let's explain that characteristics are the traits or features that something might have. So one characteristic of a solid is that it might be hard. Pretty straight forward.
One of the main characteristics of solids is that they hold their own shape. So if you put a solid in a container, it will not change its shape, no matter how much you move or slide it around.  You can even grind a solid up, so that it fills up a container, but if you look at the powder under a microscope you will still see little tiny solids that you couldn't change. You know that liquids are different because if you put a liquid into a container, it will fill it up as much of the container as it can. You can even grind a solid up, so that it fills up a container, but if you look at the powder under a microscope you will still see little tiny solids that you couldn't change. You know that liquids are different because if you put a liquid into a container, it will fill it up as much of the container as it can.
 In the same way that a solid holds its shape, the atoms inside of a solid are not allowed to move around much. This is a physical characteristic of all solids. It happens no matter how small the piece of solid. The atoms in liquids and gases move around in all directions, but the solid atoms and molecules are trapped in their places. The atoms still spin and the electrons still move but the entire atoms really don't go anywhere. They just kind of jiggle in place. In the same way that a solid holds its shape, the atoms inside of a solid are not allowed to move around much. This is a physical characteristic of all solids. It happens no matter how small the piece of solid. The atoms in liquids and gases move around in all directions, but the solid atoms and molecules are trapped in their places. The atoms still spin and the electrons still move but the entire atoms really don't go anywhere. They just kind of jiggle in place.
SO YOU WANT TO BE A SOLID
Obviously, not everything is a solid. If you look around you'll see solids, liquids, and especially gases (remember the air around you). Sometimes liquids feel a physical need to become a solid. Then look out, phase changes are about to happen.
Scientists use something called a FREEZING POINT to measure when a liquid turns into a solid. There are physical effects that can change the freezing point. Pressure is one of those effects. When the pressure surrounding a substance goes up, the freezing point also goes up. That means it's easier to freeze the substance than before. When it gets colder, most solids shrink in size. There are a few which expand, but most shrink.
CRYSTALS
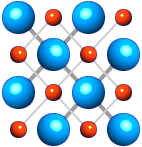 If a solid is made up of pure elements or compounds, something special happens. It can freeze into a very specific structure. This structure is called a CRYSTAL LATTICE. A crystal lattice is a very exact organization of atoms which creates a specific place for every molecule or atom in the solid. It is very neat and very compact. If a solid is made up of pure elements or compounds, something special happens. It can freeze into a very specific structure. This structure is called a CRYSTAL LATTICE. A crystal lattice is a very exact organization of atoms which creates a specific place for every molecule or atom in the solid. It is very neat and very compact.
|